What Is The Function Of A Centrosome In An Animal Cell
- Question and Answer
- Open Admission
- Published:
Q&A: Who needs a centrosome?
BMC Biology volume 11, Article number:28 (2013) Cite this article
What is the role of the centrosome?
The centrosome has several functions. The central ane is equally the major microtubule organizing eye (MTOC) in proliferating animal cells: thus, it helps to organize the microtubules that form the mitotic spindle in dividing cells, and orchestrate a wide variety of cellular processes, including cell motility, signaling, adhesion, coordination of protein trafficking by the microtubule cytoskeleton and the conquering of polarity. The centrosome has crucial links to the nucleus, the Golgi, cell to cell junctions and acto-myosin cytoskeleton that are very important in positioning information technology and thus shaping the microtubule cytoskeleton in relation to the jail cell and the organism (reviewed in [1]). The role of the centrosome in organizing cellular microtubules can differ from cell to cell and be regulated differently in dissimilar phases of the life of a cell.
How does the centrosome perform its 'organizing' part?
By stimulating the formation of microtubules and anchoring them. Microtubules are dynamic structures formed by polymerization of tubulin. In dividing cells, for example, the microtubule cytoskeleton is continuously reshaped, changing dramatically from interphase to mitosis. The dynamic beliefs of microtubules is regulated by associated proteins that can stabilize microtubules as required to class the mitotic spindle and other structures (reviewed in [2, 3]).
Although microtubules can form spontaneously from loftier concentrations of tubulin in vitro, in cells they are nucleated by specialized microtubule-nucleating proteins, some of which are associated with the centrosome. The centrosome is composed of two barrel-shaped microtubule-based organelles, the centrioles, surrounded by proteins collectively called the pericentriolar material (PCM). Proteins of both the centriole and the PCM can nucleate and anchor microtubules (reviewed in [2, 3]).
The role of the centrosome in directing cellular protein traffic depends upon the intrinsic polarity of microtubules, and microtubule-associated motor proteins that motility differentially towards i microtubule cease or the other. By nucleating microtubules, the centrosome thus determines the tracks along which different cellular components can be transported to different parts of the jail cell. It tin can too help to define the speed at which components movement along those tracks, and act as a signaling center to modify some components before they are transported to their destinations.
What's special about centrioles that distinguishes them from other microtubule-based structures?
They take a very characteristic construction, being composed in nearly cases of nine triplets of stable microtubules in a small cylindrical arrangement approximately 0.5 μm long and 0.2 μm in diameter. Besides as their nine-fold symmetry, the highly conserved structural properties of centrioles include the presence of appendages, a defined size, high stability and ability to recruit PCM components (Figure i). Centriolar characteristics determine most backdrop of the centrosome, including dynamicity, polarity and duplication. When tethered to the membrane centrioles are chosen 'basal bodies', structures that provide the template for the formation of the axoneme, the core structure that provides rigidity and mobility to cilia and flagella (Figure 1). Centriole backdrop besides determine the nine-fold symmetry of cilia and flagella, as well equally their polarity and localization and orientation at the membrane (reviewed in [2, iii]).
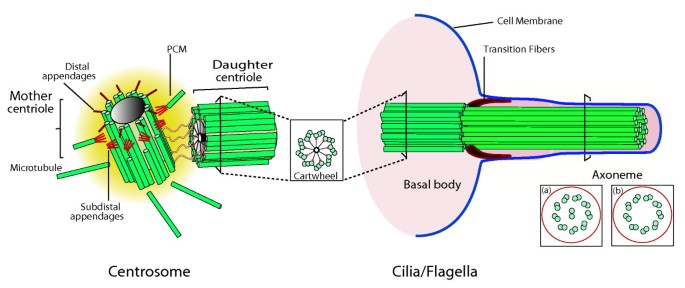
The structure of centrosomes and cilia/flagella. The centriole, also called the basal body, is a structural constituent of centrosomes, cilia and flagella. The canonical centriole has ix microtubule triplets and is approximately 0.5 μm long and 0.2 μm in diameter. Each centrosome is composed of a mother (or grandmother) and girl centriole present in an orthogonal configuration and surrounded by a matrix of proteins chosen the pericentriolar material (PCM). The older centriole (female parent) shows subdistal appendages, where microtubules are docked, and distal appendages, which are of import for docking to the prison cell membrane. In many cells the centriole migrates and tethers to the cell membrane via its appendages and seeds the growth of cilia and flagella. The skeleton of cilia and flagella, called the axoneme, results from a continuation of the basal torso structure and might be composed of 9 microtubule doublets with dynein arms and a central microtubule pair, as information technology is for most motile cilia (a); or nine doublets with no dynein arms or key pair, as it is in the case of most immotile cilia (b). The distal function of the basal body is called the transition zone, where the outer tubule stops growing. Adjusted with kind permission from Springer Science + Business organization Media: Jail cell Mol Life Sci Centrioles: active players or passengers during mitosis? 67 (2010). 2173–2194. Debec A, Sullivan Due west, and Bettencourt Dias M, Figure 1, Copyright © The Writer(s) 2010.
The ix-fold symmetry of centrioles is in part provided by the cartwheel, one of the first centriole structures that is assembled upon their biogenesis and that displays that symmetry (Figure ane) [4]. The cartwheel is composed of several components including Sas6 (Spindle assembly abnormal protein 6), which localizes to the cartwheel eye, forms oligomers that assemble into a cartwheel-hub-like structure in vitro, suggesting how the 4th structure of that poly peptide defines centriole organelle symmetry [two, 3]. This process is likely to be regulated by other molecules, such equally Ana2 (Anastral spindle two protein)/SAS5 (Spindle assembly abnormal protein 5)/STIL (SCL/TAL1 interrupting locus) (reviewed in [4, five]).
Centriole microtubules are very stable; unlike other microtubules, they are cold and detergent resistant. When labeled tubulin is injected into cells, only daughter centrioles comprise the label over a period of one cell cycle. Several centriole-specific microtubule-binding proteins, such every bit Bld10/CEP135 (Centrosomal protein of 135 kDa), SAS4 (Spindle assembly aberrant protein four)/CPAP, Centrobin and POC1 (Proteome of the centriole poly peptide ane) contribute to both stability and elongation of centriole microtubules. The overexpression of these molecules leads to longer centrioles. Other molecules, such as a depolymerizing kinesin, destabilize them. Centriole stability may as well be aided by mail-translational modifications of centriolar tubulin, such every bit glutamylation (reviewed in [5, 6]). Centriole size is highly stable and homogeneous after reaching last length, suggesting that a length-maintenance machinery must exist. Petty is known about length regulation, but information technology has been suggested that a cap may exist at the distal part of centrioles that regulates their length, through the regulation of microtubule nucleation/stabilization. The centriole component CP110 is a stiff candidate for this office: it localizes to centriole tips and its depletion leads to the formation of abnormally long centrioles that might fragment originating abnormal mitotic spindles (reviewed in [5, 6]).
What about the pericentriolar material? How do centrioles recruit it and what does information technology exercise?
Several centrosome components take been identified recently, through proteomic studies or functional genomic assay and their localization and part characterized [7–nine]. These include CEP192/SPD2 (Spindle-defective protein ii), CEP152/asl (asterless in Drosophila), Pericentrin, SAS4/CPAP and CNN (centrosomin)/CDK5RAP2 (CDK5 regulatory subunit-associated protein 2), which bind to centrioles and/or to each other and recruit microtubule nucleators, such every bit gamma-tubulin. From these studies, a new view, of a highly organized PCM is emerging, where different domains might be involved in separate functions and are regulated differently through the cell bicycle [10–14]. The size and organization of the PCM is probable to impinge on centrosome function and is adamant by the intrinsic properties of its components (size, shape and protein domains, amidst others), their availability and their regulation past kinases [5, 9, 15]. How this all works to ensure centrosome function is poorly understood and is an important avenue of inquiry for the futurity.
Aren't centrosomes essential for all cells?
No. Centrosomes are not essential in somatic cells in fruit flies, and many fauna cells don't accept them (reviewed in [16]). Virtually eukaryotic cells practice have a microtubule cytoskeleton only this can be organized in many dissimilar ways past MTOCs, which need not exist centrosomes. Several species do not accept centrosomes. In others, centrioles and/or centrosomes are absent or inactive in some tissues, while they tin exist in very high number in others. In many cells in Drosophila, centrosomes are inactive in interphase and but get active in mitosis [17]. Centrosomes are absent in many species of fungi and seed plants, as well as in many classes of protists, and in these species the specific genes encoding the proteins responsible for the nine-fold symmetry of centrioles, appendage formation, microtubule stability and length regulation accept been lost [18, 19] (Figure 2). Within animals, the flatworm Planaria, despite making centrioles that gather cilia, does not have centrosomes [xx]. Moreover, even within mammals there are cases of acentriolar cells: female oocytes lack centrioles (Effigy 3c) and the mouse embryo develops with no centrioles until the 64-jail cell stage [21]. Ordinarily, in differentiated animal cells the centrosome is no longer the major MTOC and is inactive. That is the case for muscle cells, epithelial cells and neurons. In those cell types, upon differentiation, the centrosome ofttimes loses PCM components, which delocalize to other parts of the cell such as the cytoplasmic membrane and the nuclear envelope, which then behave as the MTOC (reviewed in [16, 22]). Furthermore, centrosomes are not essential for mitotic spindle assembly, even in cells that normally have them. Moreover, mutant flies that do not assemble centrosomes from centrioles or that do non have centrioles can develop to adulthood [23, 24]. In those flies, somatic jail cell sectionalisation is fine although some defects are observed in asymmetric cell segmentation and cytokinesis [24]. In summary, centrosomes contribute to mitotic allegiance, cytokinesis and asymmetric prison cell division, merely this is not essential for the development of the flies (reviewed in [16]).
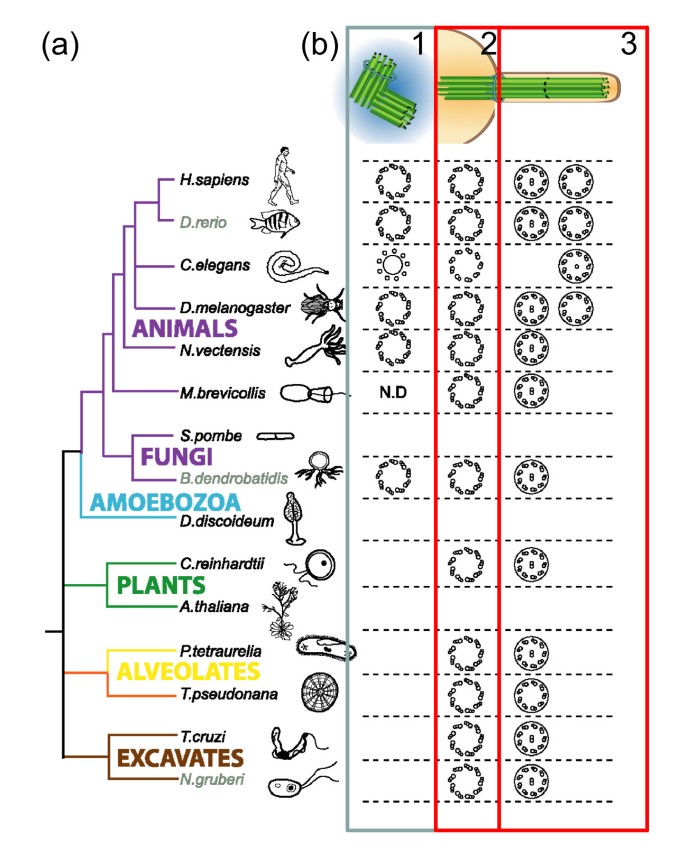
Presence of centriole/basal torso structure correlates with the presence of flagella/cilia. (a) Simplified taxonomic tree representing major eukaryotic groups in different colors. Unikonts include eukaryotic cells that, for the most function, have a single emergent flagellum divided into opisthokonts (propel themselves with a single posterior flagellum; metazoans, fungi and choanoflagellates) and Amoebozoa. Bikonts include eukaryotic organisms with two emergent flagella. Co-operative colour code: purple, opisthokonts; blueish, Amoebozoa; green, plants; yellowish, alveolates; orange, stramenopiles; rose, Rhizaria; dark-brown, excavates and discicristates. Adapted with permission [19]. (b) We represent the symmetry and number of microtubules present in centrioles/basal bodies that are either nucleating (basal body, 2) or not nucleating axonemes (centriole within centrosome, 1) and in axonemes (3) as well as the presence/absence of a fundamental microtubule pair. Note that the presence of the centriole/basal body structure (2) correlates with the presence of flagella (2 versus three) only not centrosomes (1 versus 2). Adjusted from © Carvalho-Santos et al., 2011. Originally published in J Prison cell Biol.
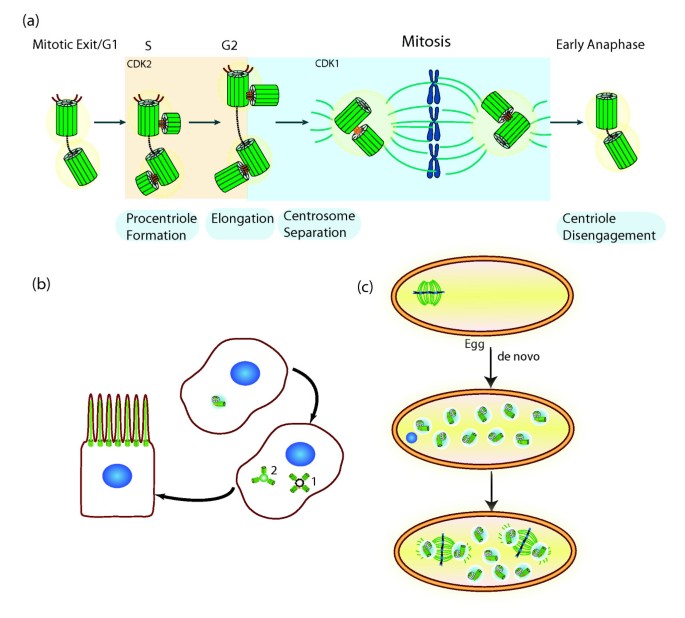
Regulation of centrosome number. (a) The canonical centrosome bike. Procentriole formation begins in S phase orthogonally to its mother. CDK2 activity may be necessary for speeding up procentriole formation and elongation, thus analogous this event with Dna replication. In G2, the girl centriole reaches full elongation and maturation with the recruitment of several molecules to the pericentriolar textile (PCM). CDK1 activity increases in G2 regulating a variety of molecules and processes needed for entry into mitosis, such every bit changes in microtubule dynamics. Through the concerted activity of molecules such as the kinase Nek2, the two centrosomes separate. The mitotic spindle segregates the chromosomes as to the two daughter cells. When a cell exits mitosis, the centrioles within the centrosome disengage. That process may permit recruitment or activation of molecules necessary for duplication. Adapted with kind permission from Springer Science + Business Media: Cell Mol Life Sci Centrioles: active players or passengers during mitosis? 67 (2010). 2173–2194. Debec A, Sullivan W, and Bettencourt Dias One thousand, figure iv, Copyright © The Writer(south) 2010. (b) Formation of multiple centrioles during ciliogenesis of epithelial cells. Hundreds of centrioles are formed either around a pre-existing mother centriole (1) or a deuterosome (two). These centrioles migrate and dock to the cell membrane, where they nucleate cilia. (c) De novo centriole germination during oogenesis of parthenogenic insect species. Centrioles disappear during oogenesis in many fauna species. Female meiosis is acentriolar. Later on egg activation multiple centrioles ascend de novo and bring together the female pronucleus resulting from meiosis. In the absenteeism of fertilization, those MTOCS prepare the first mitotic spindle in the unfertilized egg. The remaining MTOCs disappear. Adapted with permission from John Wiley and Sons: Cunha-Ferreira I, Bento I, and Bettencourt Dias 1000. Traffic. Copyright Journal compilation © 2009 Blackwell Munksgaard.
Are at that place centrosome-contained mechanisms involved in spindle associates?
In recent years it has been shown that several cooperative strategies contribute to nucleate and/or stabilize microtubules for the spindle. The chromatin pathway generates microtubules close to the chromosomes, a procedure that can depend on RanGTP or a molecular complex called CPC (chromosome rider complex). Moreover, microtubules can be nucleated from pre-existing microtubules, through a molecular complex called augmin. Finally, the nuclear envelope may too contribute to microtubule nucleation (reviewed in [25]). Therefore, it would appear that centrosomes are not always necessary for spindle assembly and cell partitioning.
Are centrosomes ever of import for cell segmentation?
Centrosomes are important for specialized cell divisions. For example, in Drosophila, developed males with no centrosomes show highly abnormal meiotic divisions [26]. Moreover, eggs from mothers that are mutant for centriole proteins arrest very early in embryonic development after only a few abnormal mitoses, showing that centrioles are necessary for syncytial mitoses [26, 27]. Moreover, disproportionate prison cell divisions can also exist abnormal in the absence of centrosomes (reviewed in [xvi]). In summary, whereas centrioles may be disposable for prison cell segmentation in some tissues of the fly, they are absolutely essential in others, perhaps due to tissue specificity constraints, such every bit weaker checkpoints, unlike cell size and/or sharing of common cytoplasm in the context of a syncytium. The aforementioned is true in other organisms, such as the Caenorhabditis elegans embryo and fission yeast, where the centrosome and its equivalent, the spindle pole trunk, are essential for bipolar spindle assembly and cytokinesis, respectively (reviewed in [sixteen, 26]).
So if information technology'southward not strictly necessary for spindle formation, what is the significance of the positioning of the centrosome at the poles of the mitotic spindle?
Equally early on as 1887, centrosomes were seen at the poles of the mitotic spindle, which led to their identification as 'the organ for cell partition' past Boveri and Van Beneden. A big argue exists on whether this is merely an epiphenomenon (reviewed in [iv]). As mentioned above, centrosomes are absent from varied eukaryotic organisms and jail cell types, suggesting they are not essential for spindle assembly (Figure 2). Pickett-Heaps said that: 'The centrioles instead appear more than likely to exist inert passengers ensured of being partitioned equally between girl cells by being fastened to the spindle apparatus'. Friedlander and Warhman proposed that 'the spindle of Metazoan cells is a basal body distributor that guarantees the authentic segregation of both chromosomes and centrioles (basal bodies)' (reviewed in [sixteen, 28]). Could this be the case? These arguments would advise that the most of import function of the centriole is to course flagella/cilia and not centrosomes; that centrosome-independent mechanisms are involved in spindle associates and that the centrosome is a modification of centrioles that only localizes centrioles at the poles of the spindle to ensure equal distribution to daughter cells.
The assay of the distribution of centriole/basal bodies, cilia/flagella and centrosomes through the eukaryotic tree of life shows there is a strict correlation between the presence of centrioles and cilia/flagella; however, the correlation is poor between the presence of centrioles and centrosomes, every bit the presence of the latter is more express in the eukaryotic tree of life (Figure two). For example, some species only form centrioles when they form cilia/flagella, such as the amoeboflagellate Naeglaeria, mosses or Planaria, in which case they class them de novo. This supports the idea that the bequeathed and nigh important function of centrioles/basal bodies is indeed in cilia/flagella assembly, and not centrosome assembly, and suggests that centriole location at the poles is an epiphenomenon (reviewed in [eighteen]). Notwithstanding, it is likely that one time at the spindle poles, centrosomes might have acquired new functions in unlike eukaryotic cells and different cell types within animals, as discussed in the previous question (reviewed in [18, 26]).
What is the evidence for the germination of the centrosome existence a strategy for equal distribution of basal bodies to the daughter cells?
The localization of centrioles at or close to the poles of the spindle is often achieved through the interaction of microtubules nucleated by the centrioles and the spindle itself. The ii centrioles in the centrosome remain associated through mitosis. The absence of a centrosome in Planaria provides food for thought. In planarians, centrosomes are never institute at the poles of the spindle [20]. Centrioles are only nowadays in epithelial cells, where they are assembled de novo and build motile cilia afterward anchoring at the prison cell membrane. Remarkably, Planaria lost from its genome several genes that are known to be involved in forming a centrosome; that is, in endowing the centriole with the power to nucleate microtubules [20]. That is the case of CNN/CDK5RAP2, SPD2/CEP192, centrobin, and NEK2 (NIMA (never in mitosis factor a)-related kinase 2); the first iii genes are important for centrosome maturation, which contributes to centrosomes' power to generate microtubules that capture the spindle (reviewed in [2, 9, 29]). A effect of their depletion is that cells inherit abnormal centriole numbers [23, 30, 31]. Furthermore, it is known that NEK2 is involved in centrosome separation, a procedure that is necessary for centrosomes to localize to opposite spindle poles at the entry to mitosis [32]. The higher up arguments suggest that the localization of centrioles at or close to spindle poles via straight microtubule nucleation or through binding to a MTOC is probable to be a strategy to ensure equal inheritance of these structures. Withal, given that the centrosome plays important roles in prison cell partition in several organisms and tissue types, information technology is possible that it has been co-opted to actively participate in spindle assembly in certain contexts [18].
What controls the number of centrioles in a cell?
Different cells accept dissimilar numbers of centrioles. While, as discussed above, most oocytes accept no centrioles, in mammalian epithelial multiciliated cells, such as the ones of the vertebrate respiratory organization, 200 to 300 basal bodies are formed in each prison cell after differentiation. Multiple centrioles form around a mother centriole, differing from the usual pattern of ane daughter centriole per mother centriole. Centrioles tin also form effectually less characterized, non-microtubule-based dense structures of heterogeneous size, called deuterosomes, whose limerick is unknown (reviewed in [4, xvi, 22]) (Effigy 3b). In a dividing cell the number of centrosomes is highly controlled through a approved duplication cycle in coordination with the chromosome wheel (Effigy 3a): one centriole forms per mother centriole in each cell cycle. Four structural steps were divers through electron microscopy in the canonical bike: separation (called disengagement) of the centrioles, formation of the daughter centrioles close to the mother, elongation of the daughters and separation of the centrosomes in G2 (reviewed in [5, vi, 9]; Figure 3a).
While a prison cell in G1 has one centrosome, during the rest of interphase and mitosis it has two, with each centrosome harboring two visible centrioles from Due south phase. In G2 the two centrosomes split up and their presence equally individual entities becomes more than obvious. Thus, when the cell enters mitosis information technology is equipped with two centrosomes, each with two centrioles, which participate in mitotic spindle assembly. The centriole cycle is regulated by the same machinery that regulates the chromosome wheel, such as cyclin-dependent kinases (Figure 3a). How those molecules regulate centriole components is not known (reviewed in [v, 6]).
Several remarkable rules regulate centriole number and localization in canonical centrosome biogenesis: it occurs once per cell cycle, only i daughter is formed per mother, and no centrioles are formed away from the mother. Once centrioles have duplicated in S phase, they cannot duplicate over again until the side by side S phase. Disengagement of the centrioles at the exit of mitosis is a prerequisite for duplication in the adjacent jail cell bike and much work is now focused on understanding this stride. Little is known nigh the control that ensures that one and merely one daughter centriole forms shut to each mother. Yet, overexpression of some regulators, such equally PLK4, SAS6 and Ana2/SAS5/STIL, can override that control [five, 6].
Centrioles can also announced without a pre-existing centriole (de novo formation). De novo biogenesis is known to occur in insect species with parthenogenic development, besides every bit in human cells upon light amplification by stimulated emission of radiation ablation of their centrosomes or overexpressing some centriole regulators (Figure 3c). The localization and number of these centrioles is non determined and can change significantly. Clearly the de novo pathway is regulated past the same molecules as the canonical pathway, all the same it has to be very well controlled to avoid multiple centriole formation in normal cells [five, six, ix].
What determines the dissimilar roles of centrioles?
Centriole age, and in consequence centrosome age, might be physiologically and developmentally very important. A consequence of the centriole bike is that each centrosome in a mitotic jail cell has a different age: one has a mother and a daughter, the other a grandmother and a daughter centriole. These differences provide variation in the competence for PCM acquisition, microtubule nucleation, anchoring and cilia formation. Afterwards cytokinesis the cell inheriting the grandmother, bagginess-harboring centriole, grows the primary cilia commencement and is thus able to respond to signaling cues, which may generate asymmetry amid those cells [33, 34].
The age of centrioles as well biases their ability to be retained differentially in Drosophila male person germ line stem cells and neuroblasts, and rodent neural progenitors, and could be implicated in proliferation and fitness of stem jail cell niches and/or progenitor cells, with consequences in evolution and morphogenesis (reviewed in [35, 36]). This topic deserves more attention, as a recent written report has shown that randomization of centrosome inheritance does not bear upon asymmetric cell sectionalisation [37]. How the age of a centriole affects its ability to be retained in one cell versus another is a very interesting question. One possibility is that centriole microtubule-nucleating and microtubule-anchoring capacity defines the population of astral microtubules associated with that centrosome and this may provide different connections with the cell cortex. Indeed, the age of the centriole determines the presence of particular proteins at each centriole, which then determines their specific microtubule nucleating capacity and centrosome inheritance [37].
Centriole and centrosome functions are clearly critical to many processes - what happens when they don't work properly?
A variety of human being diseases take been linked to centrioles and centrosomes, such as diseases of brain development, cancer and ciliopathies. The wild-blazon product of the mutated gene often localizes to centrosomes and/or has a centrosome function (for case, CPAP, CDK5RAP2, CEP152, STIL, amidst others; reviewed in [38, 39]). The well-nigh common phenotypes in brain disorders associated with those proteins are generalized disorders of growth where the brain is disproportionately affected; and the master microcephalies where the brain alone is affected and significantly reduced in size. One current hypothesis for the latter is that centrosomes help in spindle positioning in neural progenitors, contributing to a balance betwixt expansion of progenitors and generation of neurons. It is equally possible that some of the divisions with abnormal centrosomes might lead to aneuploidy and cell expiry. Animate being models of the human mutations associated with those diseases should play an important function in the understanding of their genesis (reviewed in [38, 39]).
With respect to cancer, Boveri, Hansemann and Galeotti, more than than a century ago, proposed that abnormalities in centriole duplication could be at the origin of the genome instability observed in cancer cells [39, 40]. There is an extensive debate on whether centrosome defects commonly observed in cancer, such as supernumerary centrosomes and/or centrosomes with altered construction, are a by-product of mitotic abnormalities or if they actively contribute to tumorigenesis. Centrosome abnormalities can occur early on in pre-cancerous lesions and are extensively correlated with aneuploidy, supporting a straight role for extra centrosomes in tumorigenesis [xl]. Moreover, the presence of abnormally high numbers of centrosomes per jail cell tin can generate tumors in flies [41]. How can abnormally high numbers of centrosomes generate cancer? Cancer cells arrange to dividing in the presence of supernumerary centrosomes by clustering them at the poles of a bipolar spindle; however, in the process of organizing a bipolar spindle those cells may generate aberrant chromatid attachments that lead to aneuploidy (reviewed in [39, 42]). Extra centrosomes can also interfere with disproportionate cell divisions, which may lead to hyperproliferation [41] (reviewed in [39]). Supernumerary centrioles may besides generate supernumerary cilia, which lead to abnormal ciliary signaling (for example, hedgehog), at least in tissue culture cells [33].
What about ciliopathies? Cilia can be motile or immotile, such every bit those of specialized cells similar photoreceptors, and of primary cilia, which are sensing structures that exist in virtually human cells. Motile cilia associates defects were first associated with bronchitis, sinusitis, and sperm immotility. Changes in torso symmetry have shown that ciliary movement is essential to create directional flow in the early embryo, initiating the normal left-correct developmental program. In recent years, a variety of syndromes were included in the 'ciliopathies' list, where mutations in genes whose product localizes at the chief cilia and/or centrosome pb to abnormal ciliary structure and/or office. This is the case of several rare disorders, such equally polycystic kidney disease, nephronophthisis, retinitis pigmentosa, and Bardet-Biedl, Joubert and Meckel syndromes. The study of those proteins is contributing to a better understanding of the function of immotile cilia. In particular, in several of those diseases the microtubule-based structure of the cilia is non altered, while its sensory office might exist [39, 43].
References
-
Bornens Chiliad: The centrosome in cells and organisms. Science. 2012, 335: 422-426. ten.1126/science.1209037.
-
Kitagawa D, Vakonakis I, Olieric N, Hilbert One thousand, Keller D, Olieric V, Bortfeld M, Erat MC, Flückiger I, Gönczy P, Steinmetz MO: Structural basis of the nine-fold symmetry of centrioles. Cell. 2011, 144: 364-375. ten.1016/j.cell.2011.01.008.
-
van Breugel M, Hirono G, Andreeva A, Yanagisawa HA, Yamaguchi S, Nakazawa Y, Morgner N, Petrovich 1000, Ebong IO, Robinson CV, Johnson CM, Veprintsev D, Zuber B: Structures of SAS-six suggest its organization in centrioles. Science. 2011, 331: 1196-1199. 10.1126/science.1199325.
-
Nigg EA, Stearns T: The centrosome wheel: Centriole biogenesis, duplication and inherent asymmetries. Nat Prison cell Biol. 2011, 13: 1154-1160. 10.1038/ncb2345.
-
Brito DA, Gouveia SM, Bettencourt Dias M: Deconstructing the centriole: structure and number control. Curr Opin Cell Biol. 2012, 24: 4-xiii. ten.1016/j.ceb.2012.01.003.
-
Gönczy P: Towards a molecular architecture of centriole associates. Nat Rev Mol Jail cell Biol. 2012, 13: 425-435. 10.1038/nrm3373.
-
Jakobsen L, Vanselow K, Skogs Thousand, Toyoda Y, Lundberg E, Poser I, Falkenby LG, Bennetzen G, Westendorf J, Nigg EA, Uhlen M, Hyman AA, Andersen JS: Novel asymmetrically localizing components of human being centrosomes identified by complementary proteomics methods. EMBO J. 2011, 30: 1520-1535. x.1038/emboj.2011.63.
-
Andersen JS, Wilkinson CJ, Mayor T, Mortensen P, Nigg EA, Isle of man Yard: Proteomic label of the human being centrosome by protein correlation profiling. Nature. 2003, 426: 570-574. 10.1038/nature02166.
-
Bettencourt Dias M, Glover DM: Centrosome biogenesis and function: centrosomics brings new understanding. Nat Rev Mol Cell Biol. 2007, 8: 451-463. ten.1038/nrm2180.
-
Fu J, Glover DM: Structured illumination of the interface between centriole and peri-centriolar material. Open up Biol. 2012, 2: 120104-10.1098/rsob.120104.
-
Mennella V, Keszthelyi B, McDonald KL, Chhun B, Kan F, Rogers GC, Huang B, Agard DA: Subdiffraction-resolution fluorescence microscopy reveals a domain of the centrosome critical for pericentriolar cloth organisation. Nat Cell Biol. 2012, fourteen: 1159-1168. 10.1038/ncb2597.
-
Gopalakrishnan J, Mennella V, Blachon S, Zhai B, Smith AH, Megraw TL, Nicastro D, Gygi SP, Agard DA, Avidor-Reiss T: Sas-4 provides a scaffold for cytoplasmic complexes and tethers them in a centrosome. Nat Commun. 2011, 2: 359-
-
Lawo South, Hasegan K, Gupta GD, Pelletier L: Subdiffraction imaging of centrosomes reveals college-gild organizational features of pericentriolar material. Nat Cell Biol. 2012, 14: 1148-1158. 10.1038/ncb2591.
-
Sonnen KF, Schermelleh L, Leonhardt H, Nigg EA: 3D-structured illumination microscopy provides novel insight into compages of human centrosomes. Biol Open. 2012, 1: 965-976. 10.1242/bio.20122337.
-
Decker M, Jaensch Southward, Pozniakovsky A, Zinke A, O'Connell KF, Zachariae West, Myers E, Hyman AA: Limiting amounts of centrosome material fix centrosome size in C. elegans embryos. Curr Biol. 2011, 21: 1259-1267. 10.1016/j.cub.2011.06.002.
-
Debec A, Sullivan W, Bettencourt Dias M: Centrioles: agile players or passengers during mitosis?. Cell Mol Life Sci. 2010, 67: 2173-2194. x.1007/s00018-010-0323-ix.
-
Rogers GC, Rusan NM, Peifer M, Rogers SL: A multicomponent associates pathway contributes to the formation of acentrosomal microtubule arrays in interphase Drosophila cells. Mol Biol Jail cell. 2008, 19: 3163-3178. x.1091/mbc.E07-10-1069.
-
Carvalho-Santos Z, Azimzadeh J, Pereira-Leal JB, Bettencourt Dias M: Development: Tracing the origins of centrioles, cilia, and flagella. J Cell Biol. 2011, 194: 165-175. 10.1083/jcb.201011152.
-
Carvalho-Santos Z, Machado P, Branco P, Tavares-Cadete F, Rodrigues-Martins A, Pereira-Leal JB, Bettencourt Dias M: Stepwise development of the centriole-assembly pathway. J Cell Sci. 2010, 123: 1414-1426. 10.1242/jcs.064931.
-
Azimzadeh J, Wong ML, Downhour DM, Sánchez Alvarado A, Marshall WF: Centrosome loss in the evolution of planarians. Science. 2012, 335: 461-463. ten.1126/science.1214457.
-
Courtois A, Schuh One thousand, Ellenberg J, Hiiragi T: The transition from meiotic to mitotic spindle assembly is gradual during early mammalian evolution. J Cell Biol. 2012, 198: 357-370. x.1083/jcb.201202135.
-
Cunha-Ferreira I, Bento I, Bettencourt Dias 1000: From zero to many: control of centriole number in development and disease. Traffic. 2009, x: 482-498. ten.1111/j.1600-0854.2009.00905.x.
-
Bettencourt-Dias M, Rodrigues-Martins A, Carpenter L, Riparbelli M, Lehmann 50, Gatt MK, Carmo N, Balloux F, Callaini G, Glover DM: SAK/PLK4 is required for centriole duplication and flagella development. Curr Biol. 2005, 15: 2199-2207. x.1016/j.cub.2005.xi.042.
-
Basto R, Lau J, Vinogradova T, Gardiol A, Woods CG, Khodjakov A, Raff JW: Flies without centrioles. Cell. 2006, 125: 1375-1386. 10.1016/j.cell.2006.05.025.
-
Meunier South, Vernos I: Microtubule associates during mitosis - from distinct origins to distinct functions?. J Cell Sci. 2012, 125: 2805-2814. 10.1242/jcs.092429.
-
Rodrigues-Martins A, Riparbelli M, Callaini G, Glover DM, Bettencourt Dias M: From centriole biogenesis to cellular function: centrioles are essential for cell division at critical developmental stages. Cell Bike. 2008, 7: 11-xvi. 10.4161/cc.7.ane.5226.
-
Stevens NR, Raposo AA, Basto R, St Johnston D, Raff JW: From stalk prison cell to embryo without centrioles. Curr Biol. 2007, 17: 1498-1503. 10.1016/j.cub.2007.07.060.
-
Friedländer K, Wahrman J: The spindle as a basal trunk benefactor. A study in the meiosis of the male silkworm moth, Bombyx mori. J Prison cell Sci. 1970, 7: 65-89.
-
Mahen R, Venkitaraman AR: Pattern formation in centrosome assembly. Curr Opin Cell Biol. 2012, 24: 14-23. 10.1016/j.ceb.2011.12.012.
-
Barr AR, Kilmartin JV, Gergely F: CDK5RAP2 functions in centrosome to spindle pole zipper and DNA damage response. J Cell Biol. 2010, 189: 23-39. 10.1083/jcb.200912163.
-
Giansanti MG, Bucciarelli Eastward, Bonaccorsi S, Gatti M: Drosophila SPD-2 is an essential centriole component required for PCM recruitment and astral-microtubule nucleation. Curr Biol. 2008, eighteen: 303-309. 10.1016/j.cub.2008.01.058.
-
Mardin BR, Schiebel East: Breaking the ties that bind: new advances in centrosome biology. J Cell Biol. 2012, 197: 11-18. 10.1083/jcb.201108006.
-
Mahjoub MR, Stearns T: Supernumerary centrosomes nucleate extra cilia and compromise primary cilium signaling. Curr Biol. 2012, 22: 1628-1634. 10.1016/j.cub.2012.06.057.
-
Anderson CT, Stearns T: Centriole age underlies asynchronous primary cilium growth in mammalian cells. Curr Biol. 2009, xix: 1498-1502. 10.1016/j.cub.2009.07.034.
-
Gonzalez C: Centrosome function during stem cell division: the devil is in the details. Curr Opin Prison cell Biol. 2008, 20: 694-698. 10.1016/j.ceb.2008.10.003.
-
Pelletier L, Yamashita YM: Centrosome asymmetry and inheritance during animal development. Curr Opin Jail cell Biol. 2012, 24: 541-546. 10.1016/j.ceb.2012.05.005.
-
Januschke J, Reina J, Llamazares S, Bertran T, Rossi F, Roig J, Gonzalez C: Centrobin controls mother-daughter centriole asymmetry in Drosophila neuroblasts. Nat Cell Biol. 2013, xv: 241-248. ten.1038/ncb2671.
-
Thornton GK, Woods CG: Main microcephaly: do all roads atomic number 82 to Rome?. Trends Genet. 2009, 25: 501-510. x.1016/j.tig.2009.09.011.
-
Bettencourt Dias G, Hildebrandt F, Pellman D, Woods G, Godinho SA: Centrosomes and cilia in human being disease. Trends Genet. 2011, 27: 307-315. 10.1016/j.tig.2011.05.004.
-
Zyss D, Gergely F: Centrosome function in cancer: guilty or innocent?. Trends Prison cell Biol. 2009, 19: 334-346. 10.1016/j.tcb.2009.04.001.
-
Basto R, Brunk K, Vinadogrova T, Skin N, Franz A, Khodjakov A, Raff JW: Centrosome amplification can initiate tumorigenesis in flies. Cell. 2008, 133: 1032-1042. 10.1016/j.jail cell.2008.05.039.
-
Godinho SA, Kwon M, Pellman D: Centrosomes and cancer: how cancer cells carve up with too many centrosomes. Cancer Metastasis Rev. 2009, 28: 85-98. x.1007/s10555-008-9163-half-dozen.
-
Goetz SC, Anderson KV: The primary cilium: a signalling center during vertebrate development. Nat Rev Genet. 2010, 11: 331-344. 10.1038/nrg2774.
Acknowledgements
I apologize to all the authors whose work could non be cited due to lack of space. I would like to thank Inês Bento and José Pereira-Leal for critically reading this manuscript. My laboratory and myself are funded past the Laboratório Associado de Oeiras, an EMBO Installation Grant (co-funded by FCT and Instituto Gulbenkian de Ciência), and the European Enquiry Council (ERC) nether the European Union'south Seventh Framework Programme (FP7/2010)/ERC Grant '61344-CentrioleStructNumber'.
Author information
Affiliations
Respective author
Authors' original submitted files for images
Rights and permissions
This article is published under license to BioMed Fundamental Ltd. This is an Open up Access article distributed under the terms of the Creative Eatables Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and Permissions
About this article
Cite this article
Bettencourt-Dias, M. Q&A: Who needs a centrosome?. BMC Biol 11, 28 (2013). https://doi.org/10.1186/1741-7007-xi-28
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/1741-7007-11-28
Keywords
- Principal Cilium
- Spindle Pole
- Spindle Associates
- Asymmetric Prison cell Division
- Microtubule Cytoskeleton
Source: https://bmcbiol.biomedcentral.com/articles/10.1186/1741-7007-11-28
Posted by: tathamferamplon.blogspot.com

0 Response to "What Is The Function Of A Centrosome In An Animal Cell"
Post a Comment